- Home
- Active Lawsuits
- Camp Lejeune
- Roundup
- Talcum Powder Lawsuit
- Bard PowerPort Catheters
- AFFF Exposure
- Toxic Baby Formula (NEC)
- CPAP Lawsuit
- Hair Straightner Lawsuit
- Paraquat
- PFAS Contamination Lawsuit
- Zantac Lawsuit
- Rideshare Lawsuit (Driver)
- Rideshare Lawsuit (Passenger)
- Depo-Provera Lawsuit
- Motor Vehicle Accident Lawsuit
- LDS Sex Abuse Lawsuit
- Oxbryta Lawsuits
- Sexual Abuse Lawsuit
- Roblox Addiction Lawsuit
- Blogs
- Contact Us
Oxbryta Lawsuits
- Home
- Oxbryta Lawsuits
What is Oxbryta?
Oxbryta, a drug for sickle cell disease, has faced increasing scrutiny over safety concerns. A recent study showed a high dropout rate, raising doubts about its effectiveness. Pfizer had already withdrawn Oxbryta from the market following reports of increased health risks, including vaso-occlusive crises and even death. Regulatory agencies in the U.S. and Europe have raised concerns, and lawsuits have been filed against the manufacturers, alleging failure to warn about the risks. Patients affected by Oxbryta-related complications are now seeking legal action for compensation.
Symptoms of a VOC can include:
Severe pain, often in the bones, chest, and abdomen;
Swelling in the hands and feet;
Fever;
Fatigue; and
Shortness of breath
How Do I Get Compensated?
Fill Out The Form
Fill in the form to see if you qualify.
Speak With Our Specialists
We'll review your submission quickly and then follow up with you.
Potential Compensation
Receive the justice and potential compensation that you and your loved ones deserve.

Oxbryta Lawsuits
Oxbryta Use and Increased Health Risks
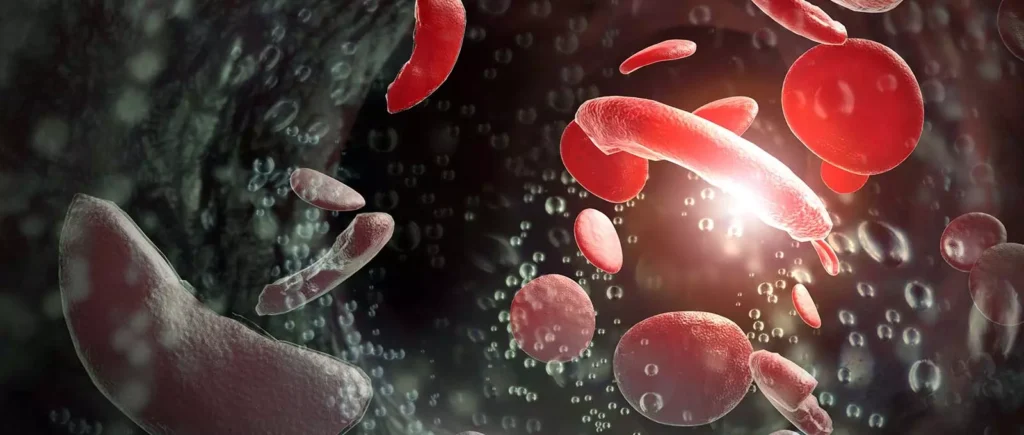
Studies have shown a possible connection between Oxbryta use and an increased risk of VOCs, a painful and dangerous complication of sickle cell disease. VOCs happen when sickled red blood cells block blood flow, causing severe pain and potentially damaging organs.
The possible link between Oxbryta and these complications raises questions about whether patients were adequately informed of the medication’s risks.
What Is a Vaso-Occlusive Crisis (VOC)?
A VOC, also known as a pain crisis, is a common and serious complication of sickle cell disease. It occurs when sickled red blood cells obstruct blood flow to tissues and organs.
